 |
| 1 |  | 
Thomson's model of the atom included negative __________ embedded in a ball of __________ charge.
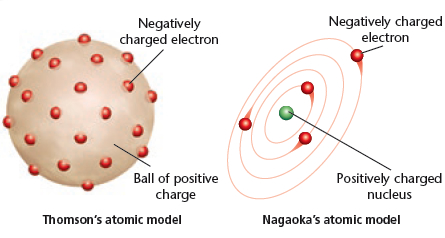 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078807239/602889/Figure_2_8.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (73.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078807239/602889/Figure_2_8.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (73.0K)</a>
|
|  | A) | protons, negative |
|  | B) | electrons, positive |
|  | C) | electrons, negative |
|  | D) | protons, positive |
|
|
 |
| 2 |  | 
Why were so few alpha particles strongly deflected in Rutherford's experiment?
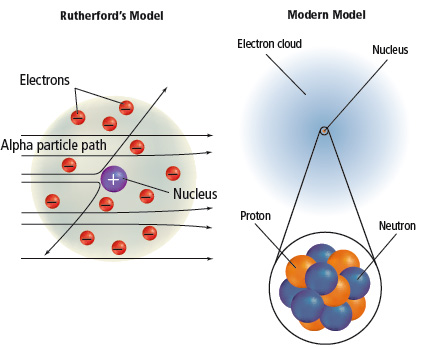 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078807239/602889/Figure_2_10.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (74.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078807239/602889/Figure_2_10.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (74.0K)</a>
|
|  | A) | The nucleus is not concentrated enough to deflect all alpha particles. |
|  | B) | Alpha particles move too quickly to be deflected very often. |
|  | C) | Alpha particles are attracted to the negative clouds of the atoms. |
|  | D) | Atomic nuclei are very small, so few alpha particles impact them. |
|
|
 |
| 3 |  | 
How were alpha particles detected in Rutherford's experiment?
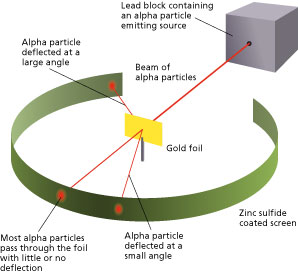 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078807239/602890/fig_2_10.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (19.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078807239/602890/fig_2_10.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (19.0K)</a>
|
|  | A) | The rays created by the moving alpha partilces were observed. |
|  | B) | The sounds of the impacting alpha particles were counted. |
|  | C) | The impacts of the alpha particles on a fluorescent screen was observed. |
|  | D) | The change in mass of the ZnS screen was measured. |
|
|
 |
| 4 |  | 
In natural waste-disposal processes, atoms are __________.
|
|  | A) | created |
|  | B) | destroyed |
|  | C) | made into aluminum |
|  | D) | recycled |
|
|
 |
| 5 |  | 
The number of protons in the nucleus of the atoms of an element determines the element's __________.
|
|  | A) | atomic number |
|  | B) | atomic mass |
|  | C) | mass number |
|  | D) | valence |
|
|
 |
| 6 |  | 
The electrons around an atom form an __________ __________.
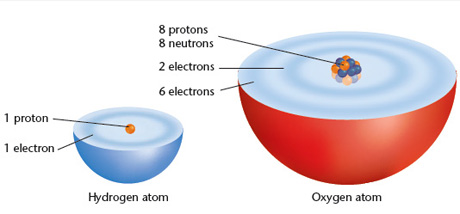 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078807239/602889/Figure_2_23.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (51.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078807239/602889/Figure_2_23.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (51.0K)</a>
|
|  | A) | electron sphere |
|  | B) | electron cloud |
|  | C) | atomic sphere |
|  | D) | atomic cloud |
|
|
 |
| 7 |  | 
These two particles are found in atomic nuclei.
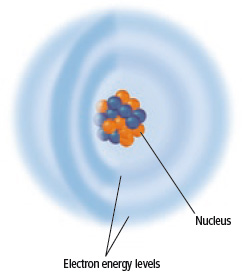 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078807239/602889/Figure_2_22.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (42.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078807239/602889/Figure_2_22.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (42.0K)</a>
|
|  | A) | protons and neutrons |
|  | B) | protons and electrons |
|  | C) | neutrons and electrons |
|  | D) | electrons and positrons |
|
|
 |
| 8 |  | 
Because light always moves at the same speed, the wavelength of light is determined by its __________.
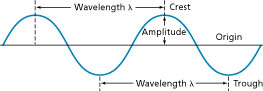 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078807239/602890/fig_2_2_q1.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (7.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078807239/602890/fig_2_2_q1.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (7.0K)</a>
|
|  | A) | amplitude |
|  | B) | crest |
|  | C) | trough |
|  | D) | frequency |
|
|
 |
| 9 |  | 
What electromagnetic waves have a longer wavelength than visible light?
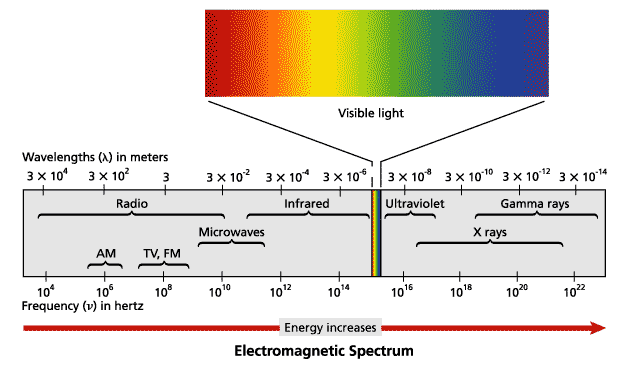 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078807239/602890/fig_2_2_q2.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (14.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0078807239/602890/fig_2_2_q2.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (14.0K)</a>
|
|  | A) | tv signals |
|  | B) | gamma rays |
|  | C) | x-rays |
|  | D) | ultraviolet |
|
|
 |
| 10 |  | 
Lavoisier came to the conclusion that air is a mixture made up mostly of these two gases.
|
|  | A) | nitrogen and oxygen |
|  | B) | oxygen and carbon dioxide |
|  | C) | hydrogen and oxygen |
|  | D) | oxygen and methane |
|
|
















